Systematic therapeutic landscape review
ISMS uses sources which are accepted by Health Technology Assessment (HTA) standards and allow fully referenced and substantiated recommendations to identify the therapeutic landscape including:
- Systematic mapping of data: identification disease attributes.
- Patient pathway identification: patient movement in health care system, including policy impact, dynamic evolutions over time including changes in Political, Economic, Social, and Technological (PEST) environment.
- Current treatment paradigm identification and optimal comparator selection.
- Identification clinical and payer parameters with differentiating improvement potential from Standard of Care (SoC).
- Stakeholder mapping: identify all relevant stakeholders, their impact on one another and role in market access.
- Health care policy clustering: evaluation of the direction of policies, thereby allowing benchmarking against other countries or World Health Organization (WHO).
Value substantiation and communication
The multilevel HEOR & market access assessment will allow to optimize strategic decisions in a broader healthcare setting and generate both clinical and economical perspective data which can be integrated in internal communication and external endorsement strategies throughout the drug development cycle including the following key value message communication tools:
GLOBAL VALUE DOSSIER
A detailed information resource containing all supporting evidence on disease context, current management of the disease, target product information, clinical value and potential of the target product, budget impact and cost-effectiveness of the product and an objection handler, in line with the product’s key value messages as outlined by the client, allowing targeted value communication to payers, clinical/scientific stakeholders, etc.


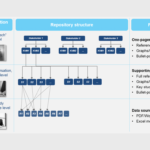
VALUE TOOLKIT
Strong communicative tool to substantiate fact-based key value messages on unmet need, clinical effect, cost-effectiveness and budget impact towards various internal and external stakeholders, structured in a 3-level communication tool consisting of one-pagers, supporting evidence and complete references to data sources. The value toolkit can be generated at different phases of drug development, and continuously updated when new clinical and/or economical data become available.


HEOR: Health Economics and Outcomes Research
Health economic modeling
Health economic models providing insight on (long-term) economical product impact are developed according to accepted HTA standards and guided with a fully referenced and substantiated technical manual.
COST-EFFECTIVENESS MODEL
Health economic model to prove cost effectiveness of a new product in comparison to standard of care with first focus on disease-specific model development with country-specific adaptations.


BUDGET IMPACT MODEL
Health economic model to quantify the potential budget impact by comparing the direct medical costs of a new product in comparison to standard of care.
