
Identification of clinical and payer parameters with differentiating improvement potential.
Evaluation of target product profile differentiation and added value compared to Standard of Care (SoC).
Health economic modeling to substantiate clinical and economical value in broad health care environment.

Systematic therapeutic landscape review of:
- Endorsed clinical guidelines (medical, societal, Health Technology Assessment (HTA)).
- HTA assessments (National Institute for Health and Care Excellence (NICE), Institute for Quality and Efficiency in Health Care (IQWiG), Hospital Accreditation Standards (HAS),…).
- Clinical trial data: long-term disease trials and Randomized Clinical Trial (RCT) therapeutics (Cochrane reviews, meta-analysis or referred to in HTA assessments).
- Label data (key countries).
- Real-life data (incl. treatment adherence).
- Market research and payer reports.
To identify:
- Disease attributes:
- Natural disease course.
- Drivers of disease progression.
- Map therapeutic environment.
- Current Standard of Care: optimal comparator selection.
- Clinical & payer stakeholder value determinants.

Clinicical and payer value drivers identified in determination unmet need are basis to create product assessment frame.
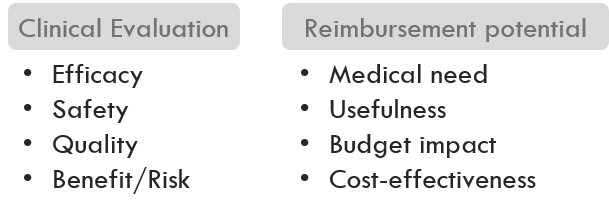
Target Product Profile (TPP) scenario development and relative value assessment compared to SoC.
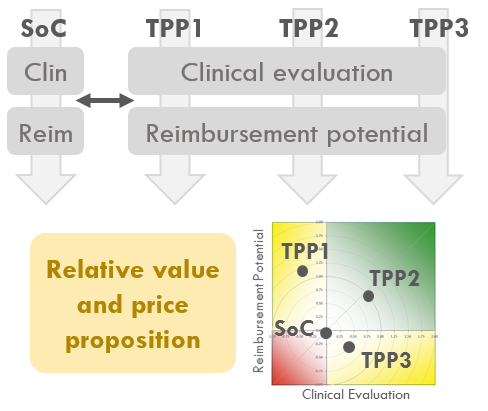

Health economic modeling comparing TPP with SoC to assess:
- Cost-effectiveness;
- Budget impact;
- Price positioning;
within broader healthcare environment including:
- Value through therapeutic impact on disease progression;
- Long-term impact including treatment adherence;
- Patient relevant outcomes.
